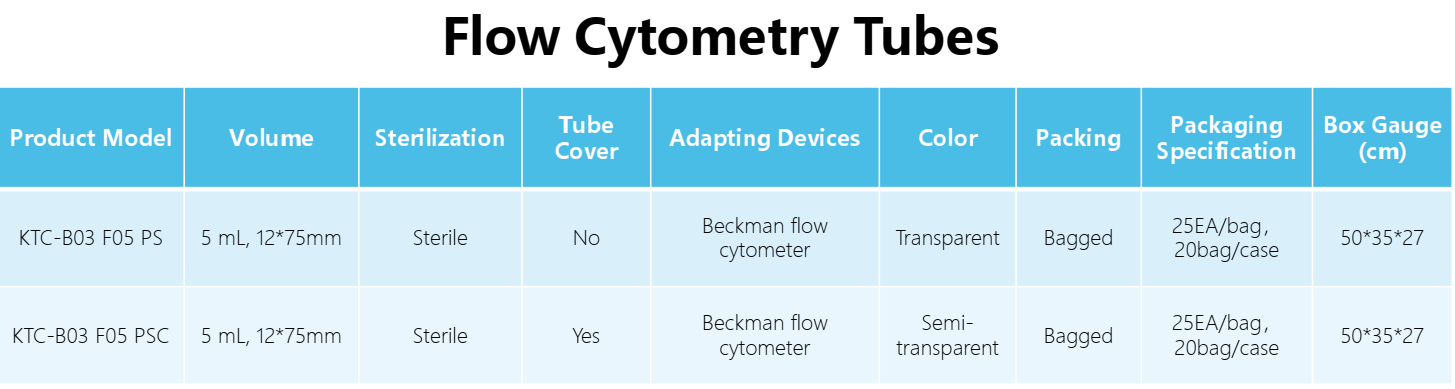
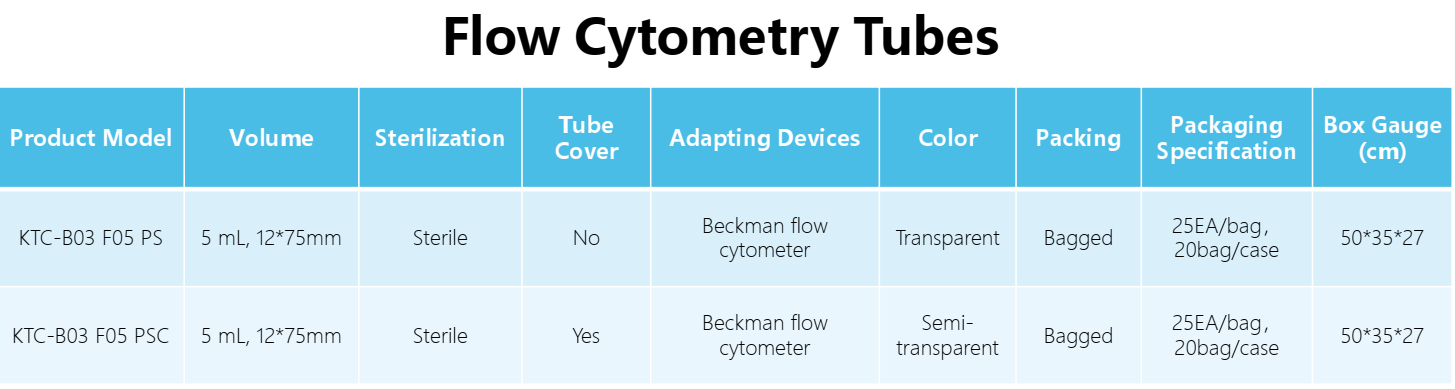
Technologyn: | Low metal content polystyrene |
Volume: | 5 mL, 12*75mm |
Tube base shape: | Round bottom |
Working temperature: | -80 ℃ ~105 ℃ |
Packaging: | 10 pieces/pack, 5 pieces/pack |
Standard: | RoHS 2.0: (EU) 2015/863, ISO 45001:2018, ISO 9001, ISO 14001:2015 |
Application
Flow cytometry tube has excellent cleanliness, meet USP VI requirements, low metal content, and good biological stability. It is made of high-purity polystyrene material, with good biocompatibility and sterile provision. The inner and outer surfaces are smooth, uniform, highly transparent, and chemically stable.
Features
●Features high transparency, anti-static stability.
●Double-locking cap with a round bottom.
●Smooth, uniform surfaces with stable chemical properties.
●Utilizes USP Class VI medical-grade materials and GMP Class 100,000 cleanroom production.
●Offers a dual-position cover with breathable and sealed settings.
●Flexible operation and preventing sample loss.
Standard Quality
●RoHS 2.0: (EU) 2015/863 Restriction of Hazardous Substances
●ISO 45001:2018 Occupational Health and Safety Management Systems
●ISO 9001:2015 Quality Management Systems
●ISO 14001:2015Environmental Management Systems
Manufacturing Accreditations
●ISO 9001 Quality Management System
●ISO 13485 Quality Management System
●QSR820 FDA Quality System Requirements
OEM Services
●Product design and development
●Flexible & timely manufacture
●Quality control and regulatory compliance
●Cost effective with product assurance
●Packaging & shipment
●Technical support
Application
Flow cytometry tube has excellent cleanliness, meet USP VI requirements, low metal content, and good biological stability. It is a sterile provision and is made of high-purity polystyrene material, with good biocompatibility. The inner and outer surfaces are smooth, uniform, highly transparent, and chemically stable.
Features
●Features high transparency, anti-static stability.
●Double-locking cap with a round bottom.
●Smooth, uniform surfaces with stable chemical properties.
●Utilizes USP Class VI medical-grade materials and GMP Class 100,000 cleanroom production.
●Offers a dual-position cover with breathable and sealed settings.
●Flexible operation and preventing sample loss.
Standard Quality
●RoHS 2.0: (EU) 2015/863 Restriction of Hazardous Substances
●ISO 45001:2018 Occupational Health and Safety Management Systems
●ISO 9001:2015 Quality Management Systems
●ISO 14001:2015Environmental Management Systems
Manufacturing Accreditations
●ISO 9001 Quality Management System
●ISO 13485 Quality Management System
●QSR820 FDA Quality System Requirements
OEM Services
●Product design and development
●Flexible & timely manufacture
●Quality control and regulatory compliance
●Cost effective with product assurance
●Packaging & shipment
●Technical support