Technologyn: | High cleanliness medical grade polystyrene |
Material: | Polypropylene |
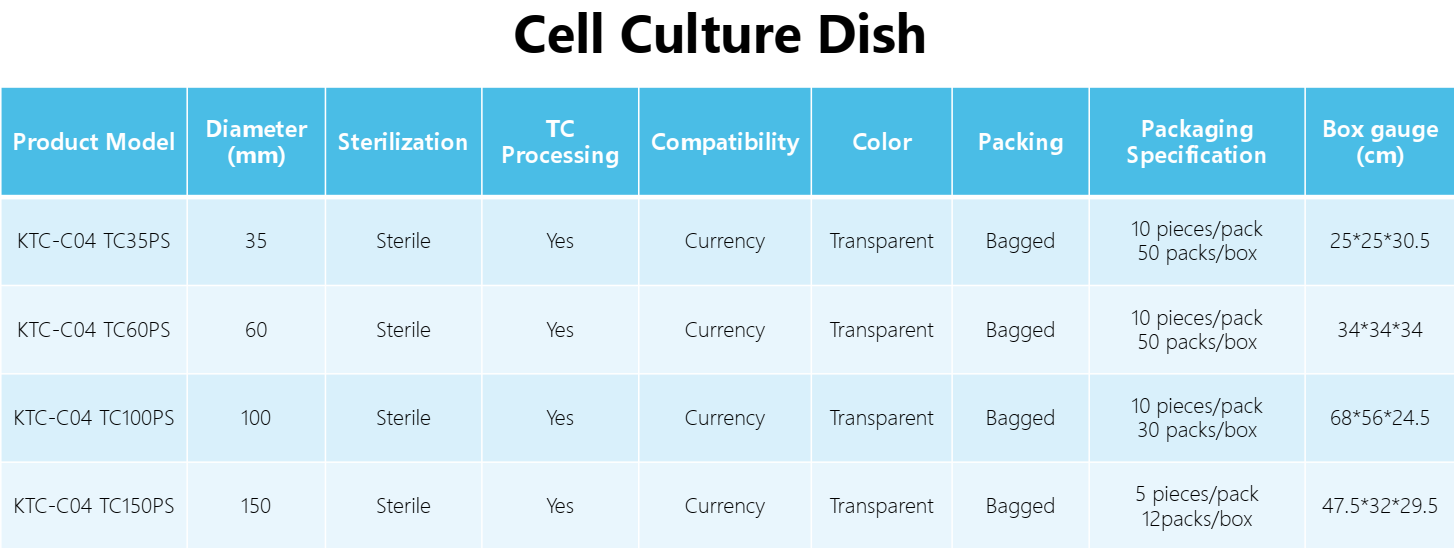
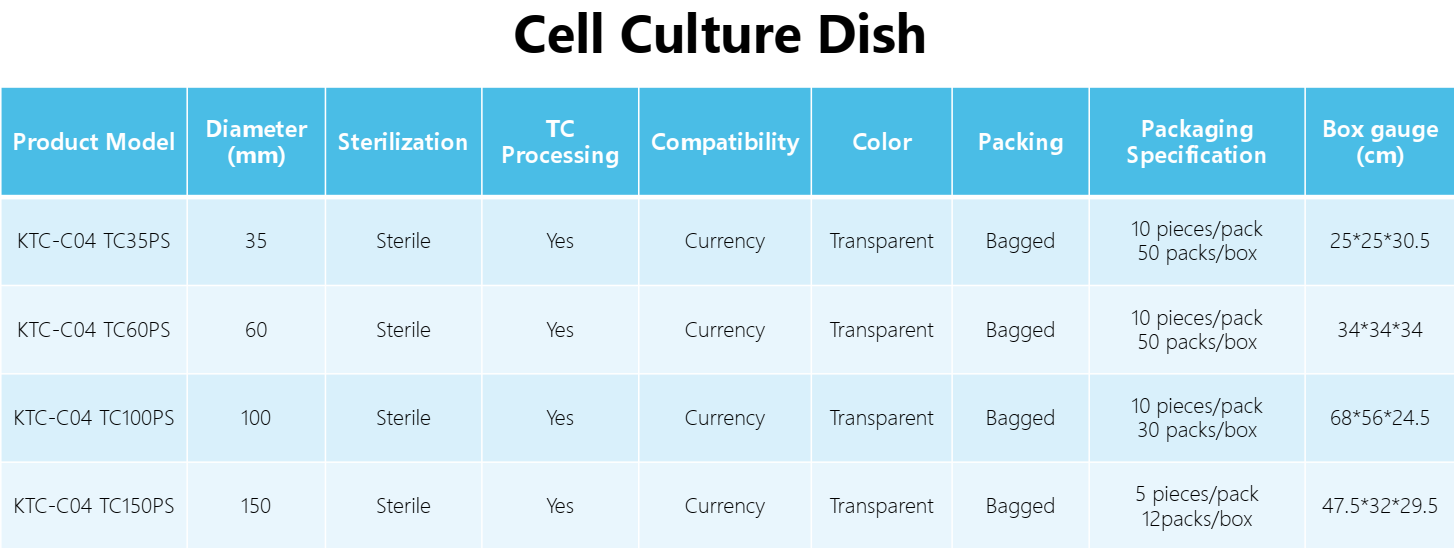
Diameter: | 35mm, 60mm, 100mm,150mm |
Working temperature: | -80 ℃ ~105 ℃ |
Packaging: | 10 pieces/pack, 5 pieces/pack |
Standard: | RoHS 2.0: (EU) 2015/863 |
Application
The culture dish is made of high-purity medical grade polystyrene material, which meets USP VI certification, has good biocompatibility, and is designed with a grip ring that is easy to operate with one hand. It is sterilized and treated with TC. The inner and outer surfaces of the culture dish are smooth, uniform, and chemically stable.
Features
●USP Class VI compliant material for excellent biocompatibility.
●Smooth, uniform surfaces with stable chemical properties.
●High transparency, with grip ring, easy to operate with one hand.
●Surface TC treatment, results in excellent cell adhesion performance.
●Sterilization by irradiation, no DNA enzyme, no RNA enzyme, no pyrogen
●Made of medical grade polystyrene material and produced in a GMP Class 10000 cleanroom.
Standard Quality
●RoHS 2.0: (EU) 2015/863 Restriction of Hazardous Substances
Manufacturing Accreditations
●ISO 9001 Quality Management System
●ISO 13485 Quality Management System
●QSR820 FDA Quality System Requirements
OEM Services
●Product design and development
●Flexible & timely manufacture
●Quality control and regulatory compliance
●Cost effective with product assurance
●Packaging & shipment
●Technical support
Application
The culture dish is made of high-purity medical grade polystyrene material, which meets USP VI certification, has good biocompatibility, and is designed with a grip ring that is easy to operate with one hand. It is sterilized and treated with TC. The inner and outer surfaces of the culture dish are smooth, uniform, and chemically stable.
Features
●USP Class VI compliant material for excellent biocompatibility.
●Smooth, uniform surfaces with stable chemical properties.
●High transparency, with grip ring, easy to operate with one hand.
●Surface TC treatment, results in excellent cell adhesion performance.
●Sterilization by irradiation, no DNA enzyme, no RNA enzyme, no pyrogen
●Made of medical grade polystyrene material and produced in a GMP Class 10000 cleanroom.
Standard Quality
●RoHS 2.0: (EU) 2015/863 Restriction of Hazardous Substances
Manufacturing Accreditations
●ISO 9001 Quality Management System
●ISO 13485 Quality Management System
●QSR820 FDA Quality System Requirements
OEM Services
●Product design and development
●Flexible & timely manufacture
●Quality control and regulatory compliance
●Cost effective with product assurance
●Packaging & shipment
●Technical support